First Experiment Explaination and Disscussion
This is my first anodization and the target pore size on the alumina membrane was 40-60nm.I used Aluminum foil that was 0.25mm and 99.99% pure that was previously electro-polished by my supervisor Gulya Korneva. A piece was cut out and attached to an anode and a stainless steel plate was used for the cathode.
The electrolyte chosen to use was a sulfuric acid solution. We decided to do two different concentrations of sulfuric acid, 0.5M and 20%, and each at a different voltage. There was some difficulty getting the voltage to my desired value in the 0.5M because the current can not go to high or else it will damage the surface of the membrane. Unfortunately the current did rise to over 1A and the power generator was immediately shut off and voltage switch lowered. The voltage was turned back on and reached 27.2V with a stable current of 0.023. Any higher and the current immediately jumped but it only crossed 1A once. The voltage chosen for the 20% solution was reached well enough because we chose a lower voltage. The temperature was 1 degree Celsius for both solutions.
Once time had expired I took the samples out of solution and rinsed with DI water.
I proceeded to remove the un-anodized portion of the aluminum sample by putting it in Cupric chloride. Once the Al had been eaten away I rinsed the sample with DI water and then put it in a 0.1M phosphoric acid solution and heated to about 45C and left it in the solution for 30 minutes. After, I soaked it in acetone to get the nail polish off. Rinsed with DI water and I had my final sample.
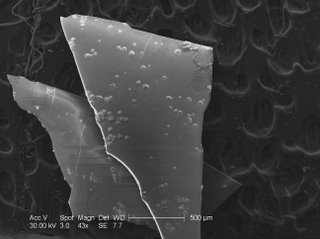
This is an SEM picture of my sample alumina membrane that was done in 0.5M sulfuric acid and unfortunately broke. You can see these dots on the surface that is due to a high current. There are no pictures for the 2 hour 20% sulfuric because no sufficient membrane was made with the desired voltage and time limit.

In closer inspection, you can see just what happens when the current is increased too much.
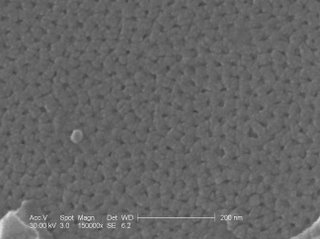
These are the nanopores that where produced from the anodization. They are between 14 and 20 nm in diameter.
Discussion: This was a good learning process. I have done the electro-polishing and it seems there are some issues concerning that which I will discuss at a later date. There are many factors that can affect the outcome of the membrane. The biggest contributors are the voltage, and temperature. Since this anodization I have done several others. Unfortunately time does not permit me to take SEM pictures of all of my membranes.


0 Comments:
Post a Comment
<< Home