Chemistry of Anodization Part2
This will probably be my last post as my co-op has ended. It was a valuable learning experience and I am sorry that I did not post more but I didn't want to be redundant. I hope this will benefit others in some way. If anyone who visits this site has any questions, feel free to ask.
I have found that each anodization is unique which is due to deviations of concentration in solution preparation and temperature. Such can be found in this research paper:
The effect of temperature and concentration on the self-organized pore formation in anodic alumina. M Almasi Kashi and A Ramazani, Institute of Physics Publishing, 38 (2005), 2396-2399.
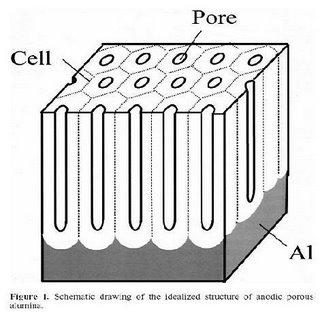 Reproduced by permission of ESC - The Electrochemical Society
Reproduced by permission of ESC - The Electrochemical SocietyConditions for fabrication of ideally ordered anodic porous alumina using pre-textured Al.
Hidetaka Asoh, Kazuyuki Nishio, Masashi Nakao, Toshiaki Tamamura, Hideki MasudaJournal of The Electrochemical Society, Vol148 (4), B152-B156, 2001
Though I can't say that all of my anodizations went perfect.... Unfortunately this happened due to the anode stirp comming in contact with the stainless steel screw. I am not sure how long it went on but the high current was enough to burn through the fiberglass circuit board.
Unfortunately this happened due to the anode stirp comming in contact with the stainless steel screw. I am not sure how long it went on but the high current was enough to burn through the fiberglass circuit board.
Happy Anodizing!

